PhyMedExp has an L2 laboratory, located at the Crastes de Paulet site. A second one is currently under construction at the IURC site (Faculty of Medicine, University of Montpellier). These L2 containment laboratories include an airlock in overpressure, and the “handling” part in depression. The air is treated by a system of HEPA filters. They are equipped with NF-certified class II biosafety cabinets, C02 laboratory incubators, an hypoxic culture chamber, a FlexCell® cell stretching bioreactor, an IONOPTIX electrostimulation system, a benchtop flow cytometer, a Neon® electroporator (Invitrogen), a CellDrop® cell counter and binocular microscopes.
The L2 laboratories are available for the various research teams at each site and to any laboratory working in collaboration with one of these entities, however with regulated access. A steering committee is made up of a member of each team, a health/safety representative and the unit director.
PhyMedExp L2 laboratories are authorized to handle samples and cells of human origin and class 2 genetically modified organisms.
To date, different cellular approaches are implemented daily:
Differentiation from human cells :
- human skeletal muscle satellite cells
- humaun iPS cells in cardiomyocytes or neurons from the same patient or healthy subject in 2D (see movie 1) or 3D culture (organoids) (Dr Albano Meli). This expertise is used at the Organoids Platform of Montpellier (POM, Biocampus) which includes the L2 PhyMedExp platform.
Human cell co-culture :
From iPS-derived cardiomyocytes and neurons, these 2 human cell types are then co-cultured to form a “patient-specific” neurocardiac organ-on-a-chip technology which is used for modeling pathologies and for pharmacological screening (movie 2)
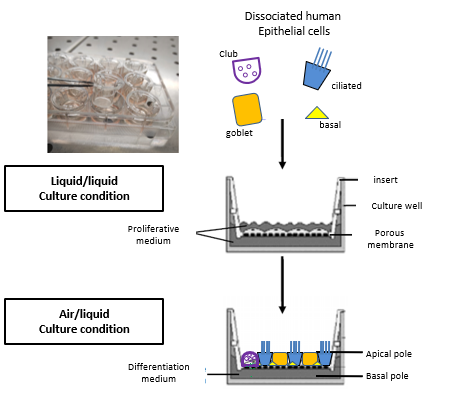
Primary culture of human bronchial and nasal epithelial cells at an air-liquid interface:
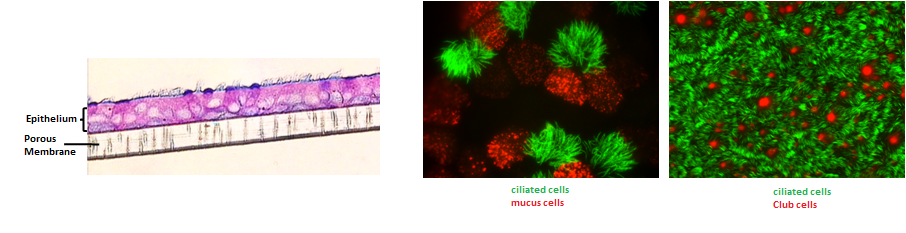
From bronchial biopsies obtained during fibroscopy or nasal cell sampling using a curette, we rebuild a pseudo-stratified, mature and functional bronchial epithelium comprising all the cell types of the proximal epithelium in vivo (ciliated cells, mucus cells, basal cells, Club cells) that retaining the phenotype of the subject from which the biopsies are taken.

Laboratoire L2
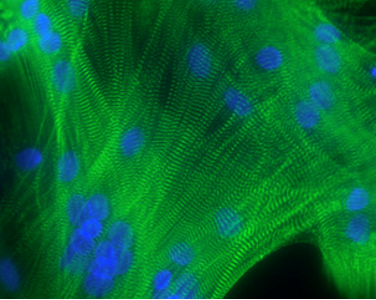
Differentiated myotubes from human satellite cells. Labeling actinin alpha (green) in Z disks and nuclei (blue)
Film 1
Film 2